AI in Life Science: Weekly Insights
Weekly Insights | April 13, 2025
In this issue:
Welcome back to your weekly dose of AI news for Life Science!
This week, we have some exciting new models lined up for you:
ProtComposer: Spatially Guided Protein Design with 3D Ellipsoids 🎯
NAStructural Database: Comprehensive dataabse of Ab and proteins 💊🧬
CTO: a large-scale benchmark for labelling clinical trial outcomes 🏥
Dive into these game-changing innovations and explore how they are transforming the biotech and healthcare landscapes!
ProtComposer: Spatially Guided Protein Design with 3D Ellipsoids 🎯
A key limitation in protein generative models is the lack of spatial control - while many models can create novel proteins, they struggle to match user-specified shapes or domain architectures. ProtComposer, developed by NVIDIA and MIT CSAIL, introduces a powerful new solution: a layout-to-structure model guided by 3D ellipsoid "blueprints" that represent where specific motifs (like helices and β-sheets) should be placed. These abstract layouts allow researchers to "sketch" protein structures before generating them, unlocking a new level of precision and creativity in protein engineering. ProtComposer enables intuitive, modular, and controllable design, while maintaining high fidelity and structural realism.
🔨 Applications:
Rational Protein Design - Allows users to control secondary structure placement, enabling the design of proteins with custom 3D architectures for specific functions.
Functional Protein Engineering - Supports the creation of proteins tailored to bind targets, catalyze reactions, or serve as therapeutic scaffolds through layout-driven control.
Interactive Educational & Research Tools - Makes protein design more interpretable and visual, useful for scientists, students, and AI researchers exploring biomolecular design.
📌 Key Insights:
Ellipsoid-Based Spatial Control - Uses ellipsoids as abstract spatial constraints, guiding the generative model to position structural elements with precision.
Improved Structural Complexity - Produces more diverse and compositionally rich proteins compared to models like Multiflow, which tend to favor simple or repetitive folds.
Superior Performance on Design Metrics - Outperforms leading models like Chroma and RFDiffusion in novelty, diversity, helicity, and overall designability - without sacrificing stability or realism.
NAStructural Database: Comprehensive dataabse of Ab and proteins 💊🧬
The intricate nature of antibody-antigen interaction modelling necessitates specialised resources to effectively navigate the vast landscape of structural possibilities. While computational methods have greatly advanced structural biology, databases tailored to antibody research offer unique value. NAStructuralDB provides a curated dataset of antibody, nanobody, and protein structures, enhanced with molecular contact information and annotations relevant to antibody-antigen interactions. By addressing challenges such as data redundancy and contact mapping, NAStructuralDB streamlines data preparation, offering an efficient framework for predictive modeling and analysis in antibody engineering and therapeutic biologics development. This resource facilitates the discovery and analysis of complex macromolecular structures, accelerating research in the life sciences.
🔨 Applications:
Drug Design - Comprehensive database can be used to identify new compounds of interest to develop new therapies.
📌 Key Insights:
Open-Source Database - Freely available online, providing curated structural data for antibody research
Comprehensive Datasets - 8 deduplicated datasets and 8 full datasets, containing structures in PDB, mmCIF, CSV, and JSON formats
Machine Learning Integration - An online platform designed to facilitate the use of these datasets with machine learning solutions, streamlining data preparation for predictive modeling in antibody engineering
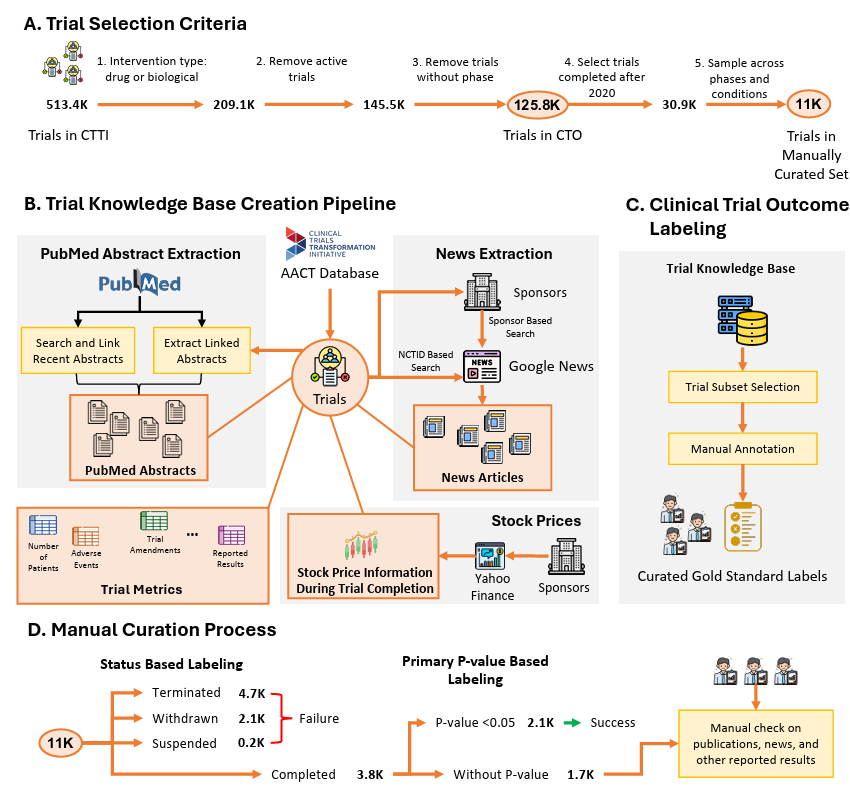
CTO: a large-scale benchmark for labelling clinical trial outcomes 🏥
Clinical trials are a costly and time-intensive phase of drug development, with a high failure rate due to inefficacy, safety concerns, and patient recruitment challenges. Their outcomes are crucial for regulatory approval and patient care, yet the scarcity of large-scale, high-quality data limits advancements in predictive modelling and evidence-based decision-making. Introducing Clinical Trial Outcomes (CTO), a fully reproducible, large-scale repository The repository integrates large language model (LLM) interpretations of trial publications, phase progression tracking, sentiment analysis from news sources, stock price movements of trial sponsors, and additional trial-related metrics.
🔨 Applications:
Condition-specific Clinical Trials Design - Facilitates the development of condition-specific clinical trial outcome prediction models by rapidly generating automated labels, significantly reducing time and effort required for manual labelling.
Pharma & Biotech Strategy: Helps companies prioritize investments by understanding patterns in trial success.
Dropout Risk Modelling: Gives insights into factors that led to high dropout rates in previous trials to adjust recruitment strategies.
📌 Key Insights:
Comprehensive modelling - Integration of LLMs to annotate & extract insights from publications and clinical trials
Prediction of clinical trial outcome - The CTO dataset significantly enhances model performance (91% F1 between ML prediction and expert curated prediction)
Comprehensive - Includes 125,000 drug and clinical trials trials
Did you find this newsletter insightful? Share it with a colleague!
Subscribe Now to stay at the forefront of AI in Life Science.
Connect With Us
Have questions or suggestions? We'd love to hear from you!
📧 Email Us | 📲 Follow on LinkedIn | 🌐 Visit Our Website