TL;DR: RFdiffusion enables atomic-level design of antibodies, validated by cryo-EM and functional assays. The pipeline (code in here) merges AI with experimental biology to create high-precision therapeutics, marking a leap forward in computational drug discovery.
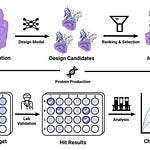
A collaborative team from the University of Washington, Children’s Hospital of Philadelphia, and other institutions has introduced a groundbreaking computational framework for de novo antibody design using RFdiffusion, a machine learning model fine-tuned to create antibodies targeting specific epitopes with unprecedented accuracy.
Key Innovations:
1. Atomically Accurate Design:
RFdiffusion generates antibody variable regions (VHHs and scFvs) that bind user-specified epitopes with structural precision. Cryo-EM validation confirmed CDR loop conformations and binding poses match computational models (RMSD <1.5 Å).
Demonstrated success across disease targets: Clostridium difficile toxin B (TcdB), influenza hemagglutinin (HA), SARS-CoV-2 RBD, and a neuroblastoma-associated peptide-MHC complex.
2. Integration with Experimental Workflows:
Combines computational design with yeast display screening and OrthoRep-based affinity maturation, boosting initial designs to single-digit nanomolar affinities while preserving binding specificity.
Validated via orthogonal methods: SPR for kinetics, cryo-EM for structural accuracy, and functional assays (e.g., TcdB neutralization in CSPG4-KO cells).
3. Multi-Chain scFv Design:
First successful de novo design of single-chain variable fragments (scFvs) with six fully designed CDR loops. Cryo-EM structures confirmed accurate heavy-light chain pairing and epitope targeting.
Combinatorial library strategies enabled identification of high-affinity binders (e.g., 72 nM for TcdB-targeting scFv6).
Applications in Drug Discovery:
Precision Targeting: Designed antibodies avoid off-target interactions (e.g., no binding to C. sordellii TcsL, despite 70% homology to TcdB).
Therapeutic Relevance: Neutralized toxins (TcdB EC50 = 460 nM), blocked viral glycoproteins (influenza HA), and targeted conserved epitopes (SARS-CoV-2 RBD).
Challenging Targets: Achieved specific binding to peptide-MHC complexes (e.g., PHOX2B:HLA-C*07:02), opening doors for cancer immunotherapies.
Limitations and Future Directions:
Success Rates: Current experimental validation requires high-throughput screening (9,000+ designs per target).
Filtering Tools: Integrating AlphaFold3 predictions could improve design selection, as retrospective analysis showed AF3 outperformed RFdiffusion in binding mode prediction.
Next Steps: Enhance glycan modeling, optimize developability (e.g., humanization), and expand to full IgG formats.
Impact on the Field:
This work shifts antibody discovery from empirical library screening to rational, epitope-specific design, accelerating therapeutic development. By combining computational power with experimental rigor, the approach enables precise targeting of undruggable epitopes and reduces reliance on animal immunization.